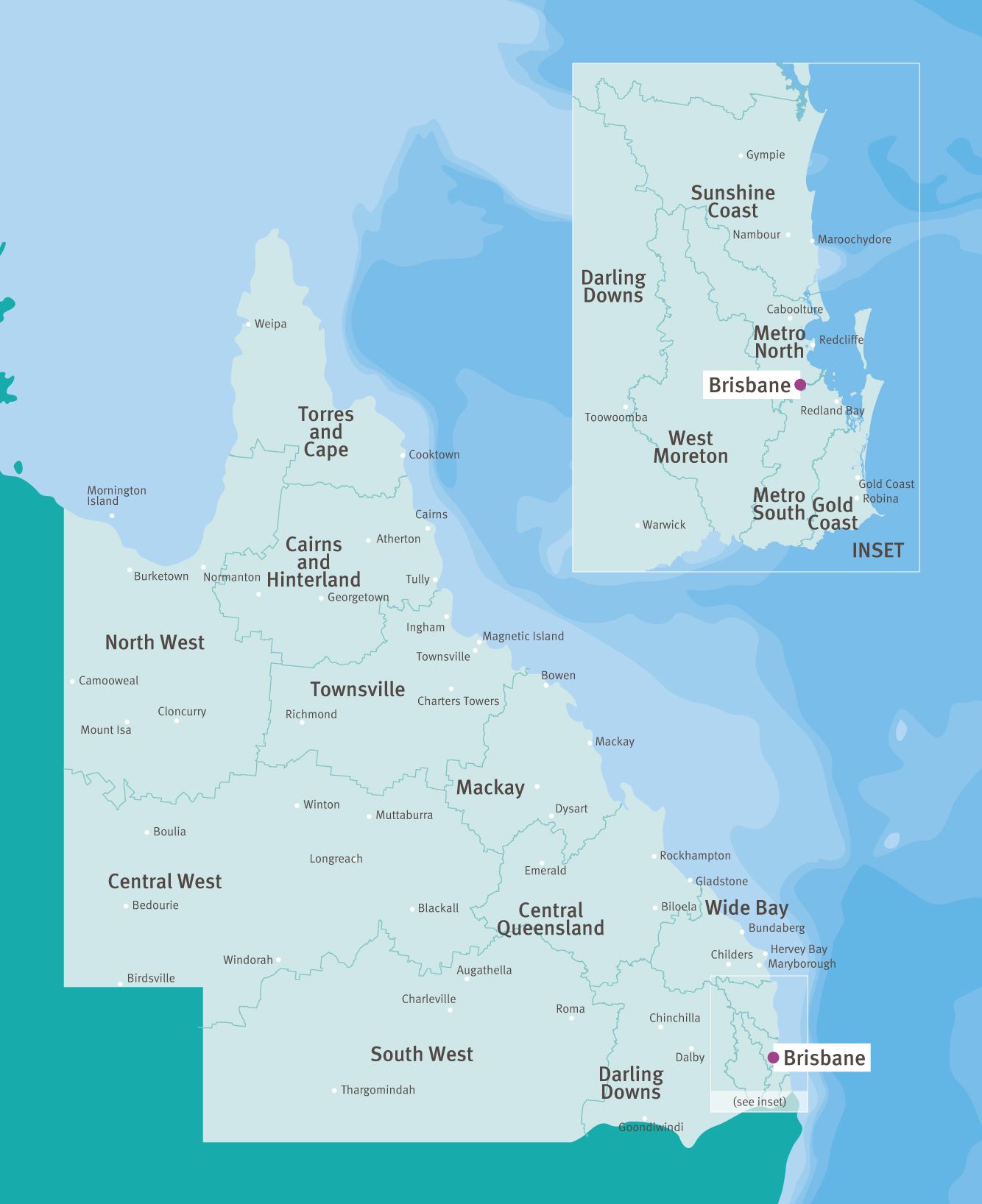
Trial sites
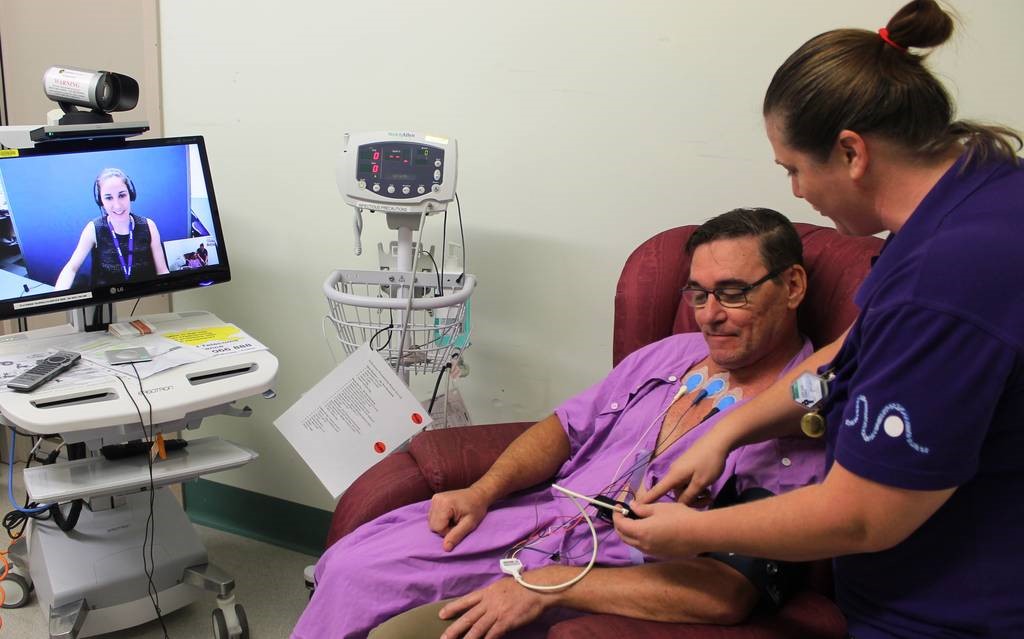
In Australia, both public and private hospitals provide access to clinical trials.

Queensland is divided into 16 Hospital and Health Services and is extremely well resourced with 122 public and 109 private hospitals.
Rural and regional health services are provided across Queensland with multipurpose and community health centres in remote areas.
There are more than 220 public sites in Queensland with access to telehealth capabilities. Our nationally acclaimed teletrials model—Australian ICH GCP (including teletrials) Standard Operating Procedures—enables potential patients to be considered for inclusion in trials.
Queensland’s small- and medium-sized businesses use technology and social media to a greater extent than the national average.
Queensland = 54%
Australian average = 48%
In Queensland, 57 per cent of people access social media every day or on most days, and 26 per cent of people check social media up to five times per day.1
One in five Australian clinical trials take place in Queensland.
Clinical trial sponsors in Australia range from small biotechs and collaborative groups to large multinational pharmaceutical companies and their contract research organisations (CROs).
Health institutions are ultimately responsible for deciding whether clinical trials take place at one of their sites.
Hospitals rely on advice about whether the proposed clinical trial complies with the principles of ethical behaviour.2
Queensland clinical practices and many aspects of its health care system are comparable to the United States, United Kingdom and most of Europe.
2 The principles of ethical behaviour are set out in the National Statement on Ethical Conduct in Human Research (2007) (updated 2018) together with an internal research governance process.
